Quality
With more than 100 million patients worldwide depending on our products, quality is crucial. It is one of our five corporate values motivating us every day.
Our commitment
to quality
We are aware of our responsibility to the health and well-being of the world’s population and are committed to complying with all national and international guidelines.
Submitting to continuous inspections, audits and quality certificates
In line with standard practice in the pharmaceutical industry, all Siegfried locations are continuously inspected for compliance with all regulations. Official inspections and customer audits, which focus on the quality of all products manufactured and supplied by Siegfried, also verify compliance with the binding rules for manufacturing, quality control and logistics processes, ensuring consumer health.
Analysis certificates, concerning appearance, purity, content, physical characteristics and GMP-conforming production of our products, are also a requirement. In addition, customers and regulatory authorities require data and certificates concerning genotoxic impurities, freedom of heparin adulteration, freedom of hidden genetically modified parts including TSE / BSE, melamine, benzene, etc. Siegfried works in close cooperation with the US-FDA concerning product piracy in order to combat counterfeiting and to safeguard consumer safety.
Ensuring sustainability and quality in the supply chain
Maintaining sustainability standards in the delivery chain adds value for our clients, helps implement our Code of Business Conduct and minimizes risk. Securing sustainability along the entire delivery chain requires cooperation and focus on long-term actions that provide enduring value.
We have clear rules when it comes to choosing and qualifying manufacturers and suppliers in order to ensure sustainability in the delivery chain. We make use of an audit system to carry out qualifications in accordance with applicable quality standards. We consider cooperation with our suppliers to be an opportunity to integrate sustainability into the entire value-added process. All our suppliers must share our principles, as they play a decisive part in our sustainability performance.
Audits are carried out periodically at our suppliers’ locations to monitor the social and ecological effects along the value-added chain, identifying potential risks and how to manage them. Non-compliance with minimum standards will result in a delivery block or change of supplier.
Adhering to strict regulation of distribution
Our aim is to build long-term relationships with our customers by providing them high-quality products and services. Distribution at Siegfried is strictly regulated and subject to relevant laws. The group-wide Code of Conduct makes no allowance for violation of the law and requires strict adherence to anti-corruption guidelines and antitrust legislation.
Good Distribution Practice (GDP), introduced following new international guidelines in 2013, aims to ensure that the entire supply chain of materials from manufacturers and suppliers to Siegfried, and from Siegfried to our customers, is safeguarded against incorrect transportation and storage conditions, as well as fraudulent attempts at counterfeiting.
Quality compliance model
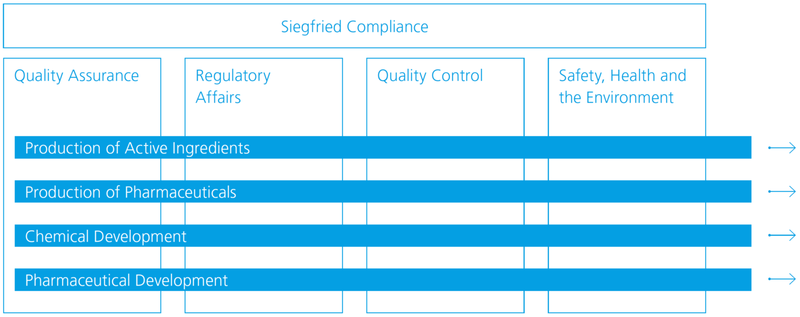
Siegfried’s compliance model is based on four parts: Quality Assurance, Regulatory Affairs, Quality Control and Safety, Health and Environment (SHE). During production, development and manufacturing, all four components are consistently taken into account in the value chain.
Quality compliance track record
In line with standard practice in the pharmaceutical industry, all Siegfried locations are continuously inspected for compliance with all regulations. Official inspections and customer audits, which focus on the quality of all products manufactured and supplied by Siegfried, also verify compliance with the binding rules for manufacturing, quality control, and logistics processes, ensuring consumer health.
Siegfried stands out when it comes to exceptional quality and regulatory compliance. All Siegfried sites are focused on the highest cGMP standards and are approved by national and international authorities, including the U.S. Food and Drug Administration (FDA), Swissmedic, Japan’s Pharmaceuticals and Medical Devices Agency (PMDA), Spanish Agency of Medicines and Medical Devices (AEMPS), the German Health Authority and more.